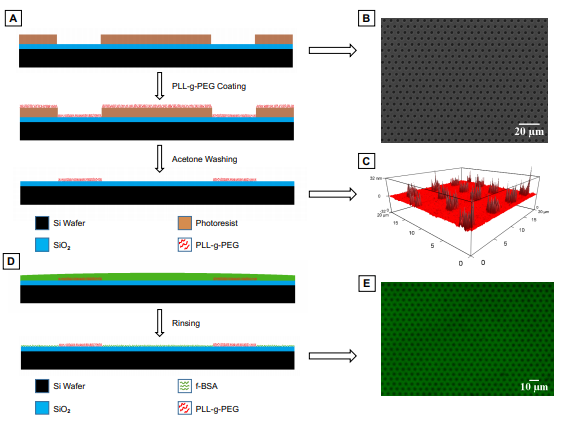
 Porous membranes are ubiquitous in cell co-culture and tissue-on-a-chip studies. These materials are predominantly chosen for their semi-permeable and size exclusion properties to restrict or permit transmigration and cell-cell communication. In a recent study, researchers fabricated micropatterned non-fouling polyethylene glycol (PEG) islands (based on PLL-g-PEI, Nanosoft Polymers) to mimic pores in order to decouple the effect of surface discontinuity from grip provided by pore wall edges. Similar to porous membranes, they found that the PEG islands hindered fibronectin fibrillogenesis with cells on patterned substrates producing shorter fibrils. Additionally, cell migration speed over micropatterned PEG islands was greater than unpatterned controls, suggesting that disruption of cell-substrate interactions by PEG islands promoted a more dynamic and migratory behavior, similarly to cells
Porous membranes are ubiquitous in cell co-culture and tissue-on-a-chip studies. These materials are predominantly chosen for their semi-permeable and size exclusion properties to restrict or permit transmigration and cell-cell communication. In a recent study, researchers fabricated micropatterned non-fouling polyethylene glycol (PEG) islands (based on PLL-g-PEI, Nanosoft Polymers) to mimic pores in order to decouple the effect of surface discontinuity from grip provided by pore wall edges. Similar to porous membranes, they found that the PEG islands hindered fibronectin fibrillogenesis with cells on patterned substrates producing shorter fibrils. Additionally, cell migration speed over micropatterned PEG islands was greater than unpatterned controls, suggesting that disruption of cell-substrate interactions by PEG islands promoted a more dynamic and migratory behavior, similarly to cells
migrating on microporous membranes. Preferred cellular directionality during migration was nearly identical between substrates with identically patterned PEG islands and micropores, further confirming disruption of cell-substrate interactions as a common mechanism behind the cellular responses on these substrates. Interestingly, cell spreading and the magnitude of migration speed was significantly greater on porous membranes compared to PEG islands with identical feature size and spacing, suggesting pore edges enhanced cellular grip. These results provide a more complete picture on how porous membranes affect cells which are grown on them in an increasing number of cellular barrier and co-culture studies. https://www.biorxiv.org/content/early/2019/02/28/563361.full.pdf.
